فيلداغلبتين
{{Drugbox | Verifiedfields = changed | verifiedrevid = 470630169 | IUPAC_name = (S)-1-[N-(3-hydroxy-1-adamantyl)glycyl]pyrrolidine-2-carbonitrile | image = Vildagliptin Structural Formulae.png | alt = Skeletal formula | image2 = Vildagliptin-3D-balls.png | alt2 = Ball-and-stick model
| tradename = | Drugs.com = International Drug Names | licence_EU = Galvus | pregnancy_category = Not recommended | legal_UK = POM | legal_status = | routes_of_administration = Oral
| bioavailability = 85% | protein_bound = 9.3% | metabolism = Mainly hydrolysis to inactive metabolite; CYP450 not appreciably involved | elimination_half-life = 2 to 3 hours | excretion = Renal
| CAS_number_Ref = ![]() | CAS_number = 274901-16-5
| ATC_prefix = A10
| ATC_suffix = BH02
| ATC_supplemental =
| CAS_number = 274901-16-5
| ATC_prefix = A10
| ATC_suffix = BH02
| ATC_supplemental =
A10BD08 (with metformin)[1]
| PubChem = 6918537
| DrugBank_Ref = قالب:علامة اختيار
| DrugBank = DB04876
| ChemSpiderID_Ref = ![]() | ChemSpiderID = 5293734
| UNII_Ref =
| ChemSpiderID = 5293734
| UNII_Ref = ![]() | UNII = I6B4B2U96P
| KEGG_Ref =
| UNII = I6B4B2U96P
| KEGG_Ref = ![]() | KEGG = D07080
| ChEMBL_Ref =
| KEGG = D07080
| ChEMBL_Ref = ![]() | ChEMBL = 142703
| ChEMBL = 142703
| C=17 | H=25 | N=3 | O=2
| molecular_weight = 303.399 g/mol
| smiles = N#C[C@H]4N(C(=O)CNC13CC2CC(C1)CC(O)(C2)C3)CCC4
| InChI = 1/C17H25N3O2/c18-9-14-2-1-3-20(14)15(21)10-19-16-5-12-4-13(6-16)8-17(22,7-12)11-16/h12-14,19,22H,1-8,10-11H2/t12?,13?,14-,16?,17?/m0/s1
| InChIKey = SYOKIDBDQMKNDQ-XWTIBIIYBW
| StdInChI_Ref = ![]() | StdInChI = 1S/C17H25N3O2/c18-9-14-2-1-3-20(14)15(21)10-19-16-5-12-4-13(6-16)8-17(22,7-12)11-16/h12-14,19,22H,1-8,10-11H2/t12?,13?,14-,16?,17?/m0/s1
| StdInChIKey_Ref =
| StdInChI = 1S/C17H25N3O2/c18-9-14-2-1-3-20(14)15(21)10-19-16-5-12-4-13(6-16)8-17(22,7-12)11-16/h12-14,19,22H,1-8,10-11H2/t12?,13?,14-,16?,17?/m0/s1
| StdInChIKey_Ref = ![]() | StdInChIKey = SYOKIDBDQMKNDQ-XWTIBIIYSA-N
| synonyms = (2S)-1-{2-[(3-hydroxy-1-adamantyl)amino]acetyl}pyrrolidine-2-carbonitrile
}}
| StdInChIKey = SYOKIDBDQMKNDQ-XWTIBIIYSA-N
| synonyms = (2S)-1-{2-[(3-hydroxy-1-adamantyl)amino]acetyl}pyrrolidine-2-carbonitrile
}}
'فيلدداغلبتين (بالإنجليزية: Vildagliptin) (سابقاً كان يعرف باسم 'LAF237 وانتشر بالأسماء التجارية 'Zomelis، 'Galvus) مضاد فموي لارتفاع السكر من (أدوية السكري) من مجموعة جديدة من الأدوية هي مثبطات DDP-4 تمنع مثبطات DDP-4 تعطيل GLP-1 البيبتيا المشابع للغلوكاغون - 1 ][2][3] and و تمنع GIP الببتيد المقوي للأنسولين المعتمد على الغلوكوز glucose-dependent insulinotropic peptide [3] by DPP-4, allowing GLP-1 and GIP to potentiate the secretion of insulin in the beta cells and suppress glucagon release by the alpha cells of the islets of Langerhans in the pancreas.
Vildagliptin has been shown to reduce hyperglycemia in type 2 diabetes mellitus.[2]
Novartis has since withdrawn its intent to submit vildagliptin to the FDA, as of July 2008.[4] The Food and Drug Administration had demanded additional clinical data before it could approve vildagliptin including extra evidence that skin lesions and kidney impairment seen during an early study on animals have not occurred in human trials.
While the drug is still not approved for use in the US, it was approved in February 2008 by European Medicines Agency for use within the EU[5] and is listed on the Australian PBS with certain restrictions.[6]
مشاركته مع الميتفورمين
وافقت وكالة الأدوية الأوربية EMEA على العلاج الفموي الذي طرحته شركة نوفارتس باسم Eucreas وهو دواء مركب من الفيداغلبتين والميتفورمين.[7]
اختطار السرطان لمثبطات DPP-4
من المعروف ان أنزيم DPP-4 معروف بعلاقته بكبت أنواع معينة من الخباثات ، وبالأخص تحديد غزو الأنسجة من الأورام، وتثبيط أنزيم DPP-4 قد يسمح بتفاقم بعض أنواع السرطان.[8][9] وقد أظهرت دراسة على تثبيط DPP-4 على سرطان الرئة غير صغير الخلايا non-small cell lung cancer - NSCLC أن وظائف DPPIV كمثبط للسرطان وتخفيضه لسرعته قد تساهم في فقد السيطرة على نمو خلايا السرطان NSCLC .[10] وهذا الاختطار الخاص بكبت السرطان عبر أنزيم DPP-4 يرتبط بكل مثبطات DPP-4 المتوفرة بالسوق كالسيتاغلبتين والساكساغلبتين.
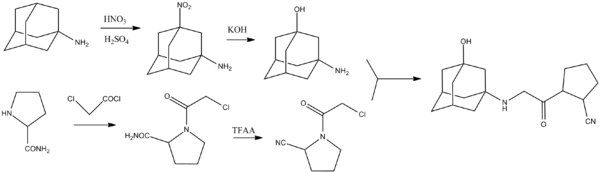
التصنيع الكيميائي
Source: 1-[[(3-Hydroxy-1-adamantyl)amino]acetyl]-2-cyano-(S)-pyrrolidine: A Potent, Selective, and Orally Bioavailable Dipeptidyl Peptidase IV Inhibitor with Antihyperglycemic Properties[11]
انظر أيضاً
مصادر
- ↑ WHO International Working Group for Drug Statistics Methodology (August 27, 2008). "ATC/DDD Classification (FINAL): New ATC 5th level codes". WHO Collaborating Centre for Drug Statistics Methodology. Retrieved 2008-09-05.
- ↑ 2٫0 2٫1 Ahrén, B; Landin-Olsson, M; Jansson, PA; Svensson, M; Holmes, D; Schweizer, A (2004). "Inhibition of dipeptidyl peptidase-4 reduces glycemia, sustains insulin levels, and reduces glucagon levels in type 2 diabetes". The Journal of Clinical Endocrinology and Metabolism. 89 (5): 2078–84. PMID 15126524. doi:10.1210/jc.2003-031907.
- ↑ 3٫0 3٫1 Mentlein, R; Gallwitz, B; Schmidt, WE (1993). "Dipeptidyl-peptidase IV hydrolyses gastric inhibitory polypeptide, glucagon-like peptide-1(7-36)amide, peptide histidine methionine and is responsible for their degradation in human serum". European journal of biochemistry / FEBS. 214 (3): 829–35. PMID 8100523. doi:10.1111/j.1432-1033.1993.tb17986.x.
- ↑ Focus on Health: Drug makers say FDA slows U.S. pipeline --- Emphasis on safety, side effects grows; swing of pendulum? By Avery Johnson and Ron Winslow 2180 words 2 July 2008 The Wall Street Journal Europe
- ↑ EU approves Novartis diabetes drug Galvus.Reuters, February 01, 2008
- ↑ NPS - better medicines, better health (August 2010). "Vildagliptin for type 2 diabetes". Retrieved 27 August 2010.
- ↑ EU approves Novartis's Eucreas diabetes drug. Reuters, February 25, 2008.
- ↑ Pro B, Dang NH (2004). "CD26/dipeptidyl peptidase IV and its role in cancer". Histol. Histopathol. 19 (4): 1345–51. PMID 15375776. Unknown parameter
|month=ignored (|date=suggested) (help) - ↑ Wesley UV, McGroarty M, Homoyouni A (2005). "Dipeptidyl peptidase inhibits malignant phenotype of prostate cancer cells by blocking basic fibroblast growth factor signaling pathway". Cancer Res. 65 (4): 1325–34. PMID 15735018. doi:10.1158/0008-5472.CAN-04-1852. Unknown parameter
|month=ignored (|date=suggested) (help) - ↑ Wesley, U; et al (2004). "Role for dipeptidyl peptidase IV in tumor suppression of human non small cell lung carcinoma cells.". Int J Cancer. 109 (6): 855–866. PMID 15027119. doi:10.1002/ijc.20091. Cite uses deprecated parameter
|coauthors=(help) - ↑ Villhauer, Edwin B.; Brinkman, John A.; Naderi, Goli B.; Burkey, Bryan F.; Dunning, Beth E.; Prasad, Kapa; Mangold, Bonnie L.; Russell, Mary E.; Hughes, Thomas E. (2003). "1-(3-hydroxy-1-adamantyl)aminoacetyl-2-cyano-(S)-pyrrolidine: a potent, selective, and orally bioavailable dipeptidyl peptidase IV inhibitor with antihyperglycemic properties.". Journal of Medicinal Chemistry. 46 (13): 2774–89. PMID 12801240. doi:10.1021/jm030091l.
روابط خارجية
- قالب:UTGlucagon – Vildagliptin
- قالب:UTGlucagon – About DPP-4
- The race to get DPP-4 inhibitors to market – Forbes.com
- Merck's March Madness – Forbes.com